What do you think about this photo?
Do you have questions or curiosities about this image? Do you want to ask something to the author, give him suggestions for improvement, or congratulate for a
photo that you really like?
You can do it by joining JuzaPhoto, it is easy and free!
There is more: by registering you can create your personal page, publish photos, receive comments and you can use all the features of JuzaPhoto.
With more than 258000members, there is space for everyone, from the beginner to the professional.
|
|
sent on 06 Aprile 2023 (14:25)Ah, ma allora questa è quasi una sfida.... Bravo Fabio, bravissimo; ho letto con passione tutto quanto ed ho preso un po' di tempo per qualche verifica (sono peggio di San Tommaso). Tutto molto bene, a mio parere. Solo un poco di ottimismo nel calcolo finale un po' troppo teorico (ma lì non hai sbagliato nulla) riguardo alla comparazione fra 4T e 2T. Ci sarebbero molte variabili, in termini motoristici da aggiungere, quali la fasatura e l'incrocio d'anticipo sulle valvole che non consentono un riempimento perfetto. Giusto per citare, alla fine degli anni '70 la potenza specifica di un motore di F1 arrivava ad approssimare un valore di 165 / 170 HP/litro a circa 13.500 r.p.m. (Ferrari). Vent'anni più tardi con "accordatura" sulle onde di risonanza si sono superati i 310 HP/litro a 19.000 r.p.m. (BMW). In vero un precedente storico ci sarebbe, quello di Honda sulla moto da GP di 50cc, 4T 2 cilindri. Sul 2T poi, la fasatura dipende dal rapporto dimensionale delle luci (larghezza x altezza), se si vuole privilegiare la potenza o se si vuole privilegiare la coppia (tipo nelle vecchie moto da "trial"). La differenza di cilindrata utile fra 2T e 4T sarebbe, a mio parere, un poco più penalizzante per il 4T perché l'aspirazione...... Meglio lasciare qui, altrimenti scriviamo un trattato di motoristica. In attinenza alla produzione di energia elettrica da fonti rinnovabili.... Ho progettato centrali di produzione elettrica con cippato di legno; energia entrante 50 MW per produzione di vapore surriscaldato fino a 114 bar e 520 °C allora premiate con certificati verdi etc. Dico solo che il rendimento effettivo, al netto degli autoconsumi (circa 1,9 Mw) si attestava al 21,6% e... tutto il resto finiva in calore scarsamente recuperabile, ovvero in quel delta entropico che ci manderà arrosto a breve. Ho progettato centrali di produzione elettrica utilizzando motori endotermici a ciclo diesel alimentate da olio vegetale (65 MW entranti) alle quali aggiunsi un particolare recupero, oltre alla turbina a vapore dai gas di scarico (22 bar erano il migliore compromesso fra qualità e quantità) un sistema di recupero per sfruttamento dei bassi valori entalpici disponibili con turbina bistadio con vettore primario avente una "campana entalpica" non simmetrica come nel 718...... Basta, per carità. Sei bravissimo, mi sarebbe piaciuto condividere la progettazione di qualche "disastro" con te... ma ormai faccio... (parola orribile) il pensionato. Un caro saluto. Paolo
Ah, ma allora questa è quasi una sfida....
Bravo Fabio, bravissimo; ho letto con passione tutto quanto ed ho preso un po' di tempo per qualche verifica (sono peggio di San Tommaso).
Tutto molto bene, a mio parere. Solo un poco di ottimismo nel calcolo finale un po' troppo teorico (ma lì non hai sbagliato nulla) riguardo alla comparazione fra 4T e 2T. Ci sarebbero molte variabili, in termini motoristici da aggiungere, quali la fasatura e l'incrocio d'anticipo sulle valvole che non consentono un riempimento perfetto. Giusto per citare, alla fine degli anni '70 la potenza specifica di un motore di F1 arrivava ad approssimare un valore di 165 / 170 HP/litro a circa 13.500 r.p.m. (Ferrari). Vent'anni più tardi con "accordatura" sulle onde di risonanza si sono superati i 310 HP/litro a 19.000 r.p.m. (BMW). In vero un precedente storico ci sarebbe, quello di Honda sulla moto da GP di 50cc, 4T 2 cilindri. Sul 2T poi, la fasatura dipende dal rapporto dimensionale delle luci (larghezza x altezza), se si vuole privilegiare la potenza o se si vuole privilegiare la coppia (tipo nelle vecchie moto da "trial"). La differenza di cilindrata utile fra 2T e 4T sarebbe, a mio parere, un poco più penalizzante per il 4T perché l'aspirazione...... Meglio lasciare qui, altrimenti scriviamo un trattato di motoristica.
In attinenza alla produzione di energia elettrica da fonti rinnovabili.... Ho progettato centrali di produzione elettrica con cippato di legno; energia entrante 50 MW per produzione di vapore surriscaldato fino a 114 bar e 520 °C allora premiate con certificati verdi etc. Dico solo che il rendimento effettivo, al netto degli autoconsumi (circa 1,9 Mw) si attestava al 21,6% e... tutto il resto finiva in calore scarsamente recuperabile, ovvero in quel delta entropico che ci manderà arrosto a breve. Ho progettato centrali di produzione elettrica utilizzando motori endotermici a ciclo diesel alimentate da olio vegetale (65 MW entranti) alle quali aggiunsi un particolare recupero, oltre alla turbina a vapore dai gas di scarico (22 bar erano il migliore compromesso fra qualità e quantità) un sistema di recupero per sfruttamento dei bassi valori entalpici disponibili con turbina bistadio con vettore primario avente una "campana entalpica" non simmetrica come nel 718......
Basta, per carità. Sei bravissimo, mi sarebbe piaciuto condividere la progettazione di qualche "disastro" con te... ma ormai faccio... (parola orribile) il pensionato.
Un caro saluto.
Paolo |
|
|
sent on 06 Aprile 2023 (14:39) | This comment has been automatically translated (show/hide original)
Paul... how much stuff! paolo, ... quanta roba! |
|
|
sent on 06 Aprile 2023 (16:27)Paolo è sempre un piacere leggere le tue note che ho copiato e incollato nel mio personale "quaderno appunti". Ti ringrazio per le precisazioni che trovo davvero tutte molto interessanti. grazie molte Paolo ! A beneficio di Simone che ha seguito il tuo bellissimo intervento posso aggiungere una ulteriore piccola nota formale: l'espressione del rendimento in forma diretta opportunamente sviluppata consente di ottenere una formula teorica della potenza di un motore. Posto il rendimento come rapporto tra la potenza sviluppata e la potenza termica messa a disposizione: ng = P / M H (M la portata di combustibile e H il potere calorifico del combustibile) indicato il rapporto massa aria - massa combustibile come a = ma / mc, possiamo introdurre il coefficiente di riempimento come rapporto tra aria aspirata e quella teoricamente aspirabile. lv = ma / (ro V) (ro è la densità dell'aria e V il volume della CC). In CC entra meno aria di quella teorica per i concetti richiamati da Paolo e per effetto delle 'perdite di carico'. Si ricava ricordando anche che il motore compie n rotazioni al minuto e che la portata è la massa nell'unità di tempo: P = ng ro V (lv/a) H n /(60 e) ng rendimento globale ro denistà dell'aria V cilindrata (volume in CC opportunamente modificato) lv coefficiente di riempimento H potere calorifico n rotazioni al minuto tutto formalmente per introdurre il concetto di coefficiente di riempimento (nei motori aspirati è chiaramente <1), quello di modulazione della potenza o di 'regolazione'. Infatti posto che la potenza chiaramente dipende dalla cilindrata V, dalla tipologia di combustibile attraverso H, attestato che: nei 2T il motore produce potenza ad ogni giro di manovella - e=1 (ciclo di 'lavaggio') nei 4T il motore produce potenza ogni 2 giri di manovella - e=2 (ciclo di pompaggio - la macchina diventa operatrice per 1 giro) se aspiri più aria puoi bruciare più combustibile e allora perché non sovra-alimentare? (conviene anche l'inter refrigerazione...intercooler) Nei cicli ad accensione comandata (benzina) la modulazione è per "quantità", cioè si strozza l'aspirazione con la valvola a farfalla diminuendo il lv, mantenendo sempre circa costante a (il rapporto in massa aria combustibile) Nei cicli ad accensione spontanea (diesel) la regolazione è per "qualità" non c'è la valvola a farfalla e si modula la quantità di combustibile che entra in CC - aspira sempre la medesima quantità di aria (circa perché occorre considerare le perdite di carico e cioè la velocità dell'aria nei condotti....) Altre considerazione ambientali a parte (vedi ciclo di lavaggio richiamato da Paolo), un vantaggio del 2T sul 4T è che, a 'partità' di cilindrata, produrrebbe una potenza doppia.
Paolo è sempre un piacere leggere le tue note che ho copiato e incollato nel mio personale "quaderno appunti". Ti ringrazio per le precisazioni che trovo davvero tutte molto interessanti.
grazie molte Paolo !
A beneficio di Simone che ha seguito il tuo bellissimo intervento posso aggiungere una ulteriore piccola nota formale:
l'espressione del rendimento in forma diretta opportunamente sviluppata consente di ottenere una formula teorica della potenza di un motore. Posto il rendimento come rapporto tra la potenza sviluppata e la potenza termica messa a disposizione: ng = P / M H (M la portata di combustibile e H il potere calorifico del combustibile)
indicato il rapporto massa aria - massa combustibile come a = ma / mc, possiamo introdurre il coefficiente di riempimento come rapporto tra aria aspirata e quella teoricamente aspirabile. lv = ma / (ro V) (ro è la densità dell'aria e V il volume della CC). In CC entra meno aria di quella teorica per i concetti richiamati da Paolo e per effetto delle 'perdite di carico'.
Si ricava ricordando anche che il motore compie n rotazioni al minuto e che la portata è la massa nell'unità di tempo:
P = ng ro V (lv/a) H n /(60 e)
ng rendimento globale
ro denistà dell'aria
V cilindrata (volume in CC opportunamente modificato)
lv coefficiente di riempimento
H potere calorifico
n rotazioni al minuto
tutto formalmente per introdurre il concetto di coefficiente di riempimento (nei motori aspirati è chiaramente <1), quello di modulazione della potenza o di 'regolazione'. Infatti posto che la potenza chiaramente dipende dalla cilindrata V, dalla tipologia di combustibile attraverso H, attestato che:
nei 2T il motore produce potenza ad ogni giro di manovella - e=1 (ciclo di 'lavaggio')
nei 4T il motore produce potenza ogni 2 giri di manovella - e=2 (ciclo di pompaggio - la macchina diventa operatrice per 1 giro)
se aspiri più aria puoi bruciare più combustibile e allora perché non sovra-alimentare? (conviene anche l'inter refrigerazione...intercooler)
Nei cicli ad accensione comandata (benzina) la modulazione è per "quantità", cioè si strozza l'aspirazione con la valvola a farfalla diminuendo il lv, mantenendo sempre circa costante a (il rapporto in massa aria combustibile)
Nei cicli ad accensione spontanea (diesel) la regolazione è per "qualità" non c'è la valvola a farfalla e si modula la quantità di combustibile che entra in CC - aspira sempre la medesima quantità di aria (circa perché occorre considerare le perdite di carico e cioè la velocità dell'aria nei condotti....)
Altre considerazione ambientali a parte (vedi ciclo di lavaggio richiamato da Paolo), un vantaggio del 2T sul 4T è che, a 'partità' di cilindrata, produrrebbe una potenza doppia.
|
|
|
sent on 06 Aprile 2023 (16:31) | This comment has been translated
 |
|
|
sent on 06 Aprile 2023 (16:36) | This comment has been automatically translated (show/hide original)
Forgot.... The more high revolutions the more power you theoretically produce.... but the faster the air moves in the ducts.... (other problems)... in short, they are machines in my opinion in which you really need a lot of skill to extract the best .... and anyway in MCI are a slave .... I may have missed a few steps... for serious questions ask Paolo :-P dimenticavo....più giri alto più potenza teoricamente produci....ma più velocemente si muove l'aria nei condotti....(altri problemi)...insomma sono macchine a mio parere in cui occorre davvero tanta maestria per estrarne il meglio....e comunque nei MCI sono una schiappa....potrei aver sbagliato qualche passaggio...per domande serie rivolgetevi a Paolo |
|
|
sent on 06 Aprile 2023 (18:35) | This comment has been automatically translated (show/hide original)
Exaggerated!
Great engineer, you are very good, it is a pleasure to read you (always) but these topics attract me like a fly on jam.
Again many congratulations, Fabio. Esagerato !
Grande ingegnere, sei bravissimo, è un piacere leggerti (sempre) ma questi argomenti mi attirano come una mosca sulla marmellata.
Ancora tanti complimenti, Fabio. |
|
|
sent on 21 Aprile 2023 (21:48) | This comment has been automatically translated (show/hide original)
And yes thanks I had not noticed the correction of the phone ....
Of course it is the lower one.
Hs - Hi = r x M(H2O)
It should be remembered that if the fuel contains sulphur, the reactions lead to the formation of a mixture that condenses in the MCI giving rise to a corrosion phenonene at low temperature.
S + O2 -> SO2 SO2 +
1/2 O2 -> SO3
SO3 + H2O -> H2SO4 E si grazie non mi ero accorto della correzione del telefono....
Naturalmente è quello inferiore.
Hs - Hi = r x M(H2O)
Occorre ricordare che se il combustibile contiene zolfo le reazioni portano alla formazione di una miscela che condensa nei mci originando un fenoneno di corrosione a bassa temperatura.
S + O2 -> SO2
SO2 + 1/2 O2 -> SO3
SO3 + H2O -> H2SO4 |
|
|
sent on 22 Aprile 2023 (0:26) | This comment has been automatically translated (show/hide original)
The stick ratio should also be taken into account because, for example, if little oxygen enters the carbon does not bind and therefore is spat out without transforming (improper term) into CO2 and / or CO. Bisognerebbe tener conto anche del rapporto stecchiometrico perchè se ad esempio entra poco ossigeno il carbonio non si lega e quindi viene sputato fuori senza trasformarsi (termine improprio) in CO2 e/o CO. |
|
|
sent on 22 Aprile 2023 (8:22)Grazie Old_pentax, prima di tutto per la tua attenzione e poi per le tue osservazioni che mi fanno molto piacere. Per chi pazientemente leggesse anche i commenti aggiungo a quanto accennato che nei 2T il processo di espulsione, affidato al ciclo di lavaggio , nel caso reale è intermedio tra due ipotesi limite. La perfetta espulsione in cui la nuova 'carica' spinge all'esterno i gas senza mescolarsi ad essi, come fosse uno stantuffo fluido, e la perfetta miscelazione in cui la nuova 'carica' si mescola con i gas da espellere ed in parte esce con essi, senza subire processo di combustione. Tutto ciò determina un rendimento di lavaggio e la definizione del coefficiente di lavaggio che, anche ad intuito, permette di definire la quantità di 'carica' da inviare per ottenere una buona espulsione dei gas combusti dalla CC. Tale coefficiente di lavaggio sempre >1 dipende dalla disposizione delle luci e dalla direzione di ingresso ed uscita dei fluidi. Per quanto riguarda il processo di combustione mi hai fatto venire in mente, sempre che io trovi il modo di allegarlo ad una immagine, un altro piccolo calcolo. Per il momento basterà dire che il carbonio ha un primo stadio di ossidazione C + 1/2 O2 -> CO che sviluppa (vado a memoria quindi devo verificare) 2350 Kcal/Kg e quindi rispetto agli 8050 Kcal/Kg della combustione completa comporta una perdita energetica rilevante. La quantità di CO dipende sia dell'eccesso di aria (ma nei MCI ad accensione comandata siamo nello stechiometrico quindi non c'è aria in più) sia da quanto è ridotto in minuscole goccioline (più sono piccole più è vantaggioso il rapporto superficie/volume) il combustibile. Viene da se che la lettura dell'O2 nei gas di scarico è una spia di una combustione non completa.....Proseguendo si potrebbe sommare alla perdita per 'imperfetta combustione' quella per 'calore sensibile'...., e così tutte le quantità che costituiscono la "perdita di rendimento"; ovviamente tutto si riconduce al trasformare la forma diretta del rendimento in quella indiretta, piccolo calcolo che per non tediare oltre lascio da parte  .
Grazie Old_pentax, prima di tutto per la tua attenzione e poi per le tue osservazioni che mi fanno molto piacere.
Per chi pazientemente leggesse anche i commenti aggiungo a quanto accennato che nei 2T il processo di espulsione, affidato al ciclo di lavaggio , nel caso reale è intermedio tra due ipotesi limite. La perfetta espulsione in cui la nuova 'carica' spinge all'esterno i gas senza mescolarsi ad essi, come fosse uno stantuffo fluido, e la perfetta miscelazione in cui la nuova 'carica' si mescola con i gas da espellere ed in parte esce con essi, senza subire processo di combustione. Tutto ciò determina un rendimento di lavaggio e la definizione del coefficiente di lavaggio che, anche ad intuito, permette di definire la quantità di 'carica' da inviare per ottenere una buona espulsione dei gas combusti dalla CC. Tale coefficiente di lavaggio sempre >1 dipende dalla disposizione delle luci e dalla direzione di ingresso ed uscita dei fluidi.
Per quanto riguarda il processo di combustione mi hai fatto venire in mente, sempre che io trovi il modo di allegarlo ad una immagine, un altro piccolo calcolo. Per il momento basterà dire che il carbonio ha un primo stadio di ossidazione C + 1/2 O2 -> CO che sviluppa (vado a memoria quindi devo verificare) 2350 Kcal/Kg e quindi rispetto agli 8050 Kcal/Kg della combustione completa comporta una perdita energetica rilevante. La quantità di CO dipende sia dell'eccesso di aria (ma nei MCI ad accensione comandata siamo nello stechiometrico quindi non c'è aria in più) sia da quanto è ridotto in minuscole goccioline (più sono piccole più è vantaggioso il rapporto superficie/volume) il combustibile.
Viene da se che la lettura dell'O2 nei gas di scarico è una spia di una combustione non completa.....Proseguendo si potrebbe sommare alla perdita per 'imperfetta combustione' quella per 'calore sensibile'...., e così tutte le quantità che costituiscono la "perdita di rendimento"; ovviamente tutto si riconduce al trasformare la forma diretta del rendimento in quella indiretta, piccolo calcolo che per non tediare oltre lascio da parte . . |
|
|
sent on 22 Aprile 2023 (10:06)Il calcolo della massa di comburente utilizzato ti permette di conoscere la quantità di ossigeno in entrata e quindi quello che potenzialmente si lega al C del combustibile. Di conseguenza hai la verifica del calcolo che hai fatto tu. Anche perché la massa di comburente per il processo è sempre quella ma in termini di volume cambia molto a causa della densità dell'aria. Ad esempio: se sali di quota l'aria è più rarefatta e la pressione atmosferica si riduce Idem se la temperatura dell'aria è maggiore Per esempio l'umidità relativa e anche la densità dell'aria di notte è maggiore rispetto al giorno perché la temperatura è inferiore. Se ho tempo ti allego i grafici di temperatura ambiente e umidità relativa dove vedrai che i due parametri sono inversamente proporzionali in modo quasi perfetto. www.ellabitalynews.it/2018/04/umidita-assoluta-e-relativa/ Tant'è che i motori a combutione interna con l'aumentare della quota diminuiscono di potenza. La cosa migliora un pochino con il compressore volumetrico di cui erano dotati gli aerei della seconda guerra mondiale. it.m.wikipedia.org/wiki/Westland_Whirlwind Anche in quel caso oltre ad una certa quota il motore perdeva potenza. Comunque uno Spitfire superò una quota di tangenza di m 15.000. Se cerchi sia sui libri di ingegneria che su internet trovo le tabelle prefatte con le perdite di potenza in funzione della quota. In sostanza: tagliare un prato sul Machu Picchu è un'impresa ardua. 
Il calcolo della massa di comburente utilizzato ti permette di conoscere la quantità di ossigeno in entrata e quindi quello che potenzialmente si lega al C del combustibile. Di conseguenza hai la verifica del calcolo che hai fatto tu.
Anche perché la massa di comburente per il processo è sempre quella ma in termini di volume cambia molto a causa della densità dell'aria.
Ad esempio:
se sali di quota l'aria è più rarefatta e la pressione atmosferica si riduce
Idem se la temperatura dell'aria è maggiore
Per esempio l'umidità relativa e anche la densità dell'aria di notte è maggiore rispetto al giorno perché la temperatura è inferiore.
Se ho tempo ti allego i grafici di temperatura ambiente e umidità relativa dove vedrai che i due parametri sono inversamente proporzionali in modo quasi perfetto.
www.ellabitalynews.it/2018/04/umidita-assoluta-e-relativa/
Tant'è che i motori a combutione interna con l'aumentare della quota diminuiscono di potenza.
La cosa migliora un pochino con il compressore volumetrico di cui erano dotati gli aerei della seconda guerra mondiale.
it.m.wikipedia.org/wiki/Westland_Whirlwind
Anche in quel caso oltre ad una certa quota il motore perdeva potenza.
Comunque uno Spitfire superò una quota di tangenza di m 15.000.
Se cerchi sia sui libri di ingegneria che su internet trovo le tabelle prefatte con le perdite di potenza in funzione della quota.
In sostanza: tagliare un prato sul Machu Picchu è un'impresa ardua.

|
|
|
sent on 22 Aprile 2023 (10:50) | This comment has been automatically translated (show/hide original)
Attach everything you want, it is always useful. “
Machu Picchu is a difficult task „
:-D
In fact, and it is interesting that the formula of power reports in an elegantly synthetic way what you have described in a more understandable way. And that's why I mentioned it, because dozens of insights are born from a simple formula.
allega tutto ciò che vuoi, è sempre utile.
“ Machu Picchu è un'impresa ardua „

infatti, ed è interessante che la formula della potenza riporti in modo elegantemente sintetico ciò che hai descritto in modo più comprensibile. Ed è il motivo per il quale l'ho richiamata, perché da una semplice formula nascono decine di approfondimenti.
|
|
|
sent on 22 Aprile 2023 (11:15) | This comment has been automatically translated (show/hide original)
Here is the graph of temperature, relative humidity and atmospheric pressure for the last week.
P.S. maybe it would be worth opening a discussion on the subject. After all, we continue to talk about CO2 emissions, electric motors Vs. endothermic but most of those who talk about it do so only by hearsay without having the slightest knowledge of the subject.
When I look at the price lists of cars with the related consumption and CO2 emissions data published in magazines I am always puzzled by the fact that electric cars are indicated with zero CO2 emissions.
Moreover, it would be necessary to indicate the COx and NOx parameters that are more significant also because the oxidizer contains N for over 78%.
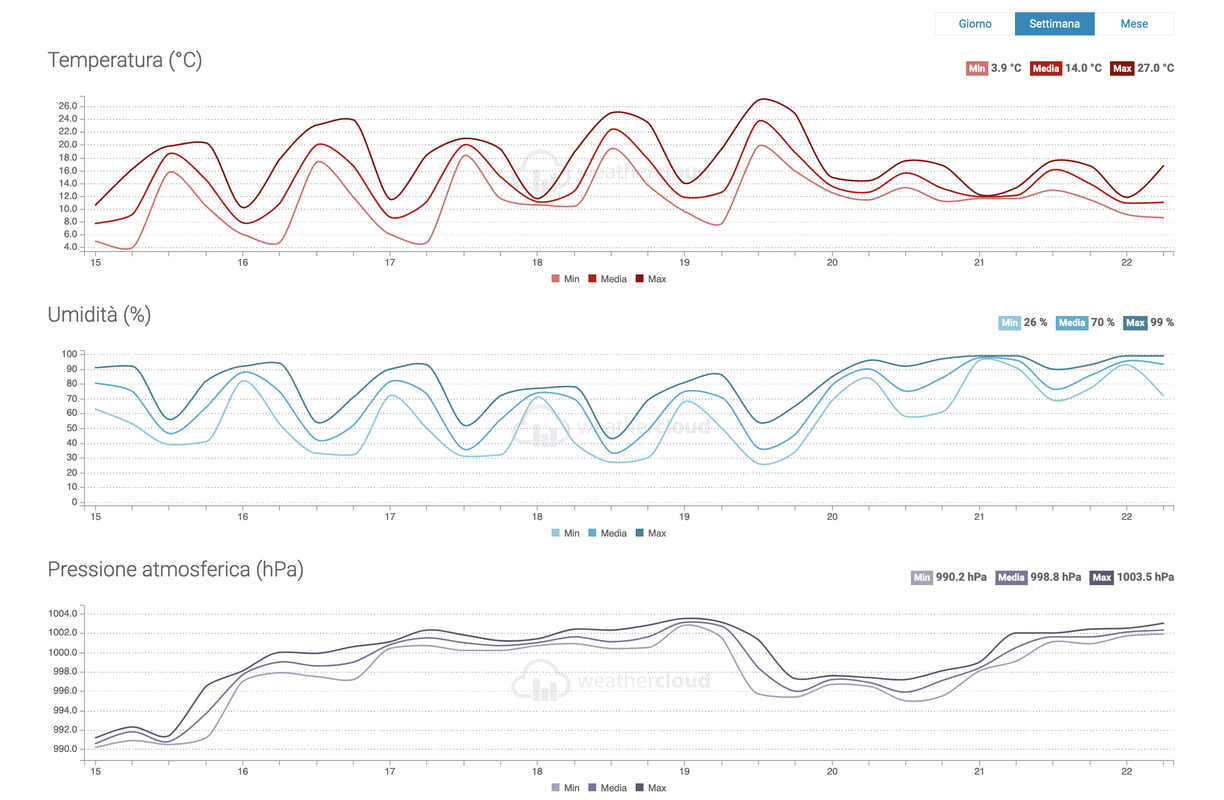
Ecco il grafico della temperatura, umidità relativa e pressione atmosferica relativo all'ultima settimana.
P.S. forse varrebbe la pena aprire una discussione sull'argomento. Del resto si continua a parlare di emissione CO2, di motori elettrici Vs. endotermici ma buona parte di coloro che ne parlano lo fanno solo per sentito dire senza avere la minima conoscenza dell'argomento.
Quando guardo i listini delle automobili con i relativi dati di consumi e emissioni di CO2 pubblicati sulle riviste rimango sempre perplesso dal fatto che le auto elettriche vengono indicate a emissioni CO2 uguale a zero.
Oltretutto bisognerebbe indicare i parametri COx e NOx che sono più significativi anche perchè il comburente contiene N per oltre il 78%.

|
|
|
sent on 22 Aprile 2023 (11:30) | This comment has been automatically translated (show/hide original)
Thank you very much Old_Pentax. Really kind. “
maybe it would be worth opening a discussion on the subject „
I don't have the courage.... :-D I would not like to be labeled here as usual ....
“ in magazines I am always puzzled by the fact that electric cars are reported to have zero CO2 emissions. „
Let's say they make a serious formal error in 'good' faith? :-D
grazie mille Old_Pentax. Davvero gentilissimo.
“ forse varrebbe la pena aprire una discussione sull'argomento „
non ne ho il coraggio.... non vorrei essere anche qui subito etichettato come il solito....Ne parlo solo con l'amico Paolo.... non vorrei essere anche qui subito etichettato come il solito....Ne parlo solo con l'amico Paolo.... Mi sembra che certi argomenti non siano graditi, meglio rimanere in clandestinità Mi sembra che certi argomenti non siano graditi, meglio rimanere in clandestinità
“ sulle riviste rimango sempre perplesso dal fatto che le auto elettriche vengono indicate a emissioni CO2 uguale a zero. „
E' un errore grave, ma sappiamo che la verità non è mai popolare.
Anzi per fortuna i 'calcoli proibiti' fatti sopra non hanno ancora attirato l'attenzione altrimenti credo che avrebbero chiuso lo spazio commenti.
I diagrammi sono molto interessanti. Chi prendesse sottomano un diagramma dell'aria umida (psicrometrico) noterà l'andamento delle curve ad umidità relativa costante ed il legame con l'umidità specifica. Una definita come rapporto tra pressioni parziali, l'altra come rapporto di masse. Ad umidità specifica costante se la temperatura della massa d'aria diminuisce, aumenta l'umidità relativa, proprio come indicato nel grafico postato da Old_pentax
|
user236140
|
sent on 06 Aprile 2024 (22:09) | This comment has been automatically translated (show/hide original)
Ahem, stuff too complex for me
but I trust what you say :-D
and to think that when I was a kid I wanted to be a nuclear engineer :-o
then I was sent back to high school in physics and I was also struggling in mate, so I gave up
on the formulas, Old_Pentax and Valgrassi know very well that I don't understand a thing and that they make me get the apecola :-D
As far as the stoichiometric ratio is concerned, I don't know what to say
, I know much better the ratio of steak with volatile fatty acids :-D
ahem, roba troppo complessa per me
ma mi fido di quello che dite 
e pensare che da ragazzo volevo fare l'ingegnere nucleare 
da bambino invece volevo fare il tranviere, quello che manovrava la leva, non il bigliettaio
poi sono stato rimandato di fisica al liceo e anche in mate facevo fatica, per cui ho lasciato perdere
per quanto riguarda la formule, Old_Pentax e Valgrassi sanno bene che non ci capisco un'acca e che a me interessa solo il risultato 
per quanto riguarda il rapporto stechiometrico non so che dire
conosco molto meglio il rapporto bisteccometrico con gli acidi grassi volatili 
|
user236140
|
sent on 06 Aprile 2024 (22:16) | This comment has been automatically translated (show/hide original)
As for EVs, I discovered Juza through the site on electric vehicles, which several years ago attracted me for the illusion that they would save me money on broth or LPG,
today they attract me like sand in a swimsuit :-D because there is no EV that is economical and with a range equal to a petrol, especially in winter; only huge vehicles, snappier and faster than a Ferrari and prohibitively expensive; so as far as I'm concerned, I'm holding on to the Baleno in soup
, however, there is no denying that EVs are quiet and don't emit a stench
per quanto riguarda gli EV, pensate che ho scoperto Juza proprio attraverso il sito sui veicoli elettrici, che parecchi anni fa mi attiravano per l'illusione che mi facessero risparmiare sulla broda o sul GPL
oggi mi attirano come la sabbia nel costume da bagno  perché non esiste alcun EV che sia economico e con autonomia pari a un benzina, specie d'inverno; solo veicoli enormi, più scattanti e veloci di una Ferrari e dai costi proibitivi, che al freddo si arenano come un'orso obeso sul pack perché non esiste alcun EV che sia economico e con autonomia pari a un benzina, specie d'inverno; solo veicoli enormi, più scattanti e veloci di una Ferrari e dai costi proibitivi, che al freddo si arenano come un'orso obeso sul pack
quindi per quanto mi riguarda, mi tengo stretta la Baleno a broda finché campa
non si può tuttavia negare che gli EV siano silenziosi e non emettano puzza
|
user236140
|
sent on 06 Aprile 2024 (22:26) | This comment has been automatically translated (show/hide original)
And what about mowers?
when I was a kid I loved the cool Flymo

I had retrieved one from a junk dealer, I had dismantled it, cleaned it, changed the bark and it was back as good as new
, you piloted it with a finger, but it made a crazy noise :-D
Today I prefer to use a wired electric one, very quiet
E per le falciatrici che dire?
da ragazzo adoravo le fichissime Flymo
vhgmc.co.uk/wp-content/uploads/2020/07/IMG_20200706_161045_resized_202
ne avevo recuperata una da un rigattiere, l'avevo smontata, ripulita, cambiati i cortecchi ed era tornata come nuova
la pilotavi con un dito, ma faceva un rumore pazzesco 
oggi preferisco usarne una elettrica a filo, molto silenziosa
|
|
|
sent on 07 Aprile 2024 (5:54) | This comment has been automatically translated (show/hide original)
Claudio, you would have been like Homer Simpson controlling a painting in some French power plant. :-)
If you put in the effort, you understand the formulas. There's nothing difficult about it.
Claudio saresti stato come Homer Simpson a controllo di un quadro in qualche centrale francese. 
Se ti impegni le formule le capisci. Non c'è niente di difficile.
|
user236140
|
sent on 07 Aprile 2024 (11:17) | This comment has been automatically translated (show/hide original)
“ If you commit to the formulas, you will understand them „
Yes, but I have to commit myself :-D
On the other hand, I completed the scientific high school and in some fields I was quite good
then at the high school they gave us a geometry problem that had no solution :-o
Despite this, I came out quite well
and then at Uni I still had to pass exams in chemistry, biochemistry, physics and statistics and even there I came out quite well
, however, today I prefer the "practical" or popularizers such as Piero Angela, who explained things very well, even the most complex ones, without using the :-D formulas
“ Se ti impegni le formule le capisci „
Sì, ma devo impegnarmi per l'appunto 
D'altronde, il liceo scientifico l'ho completato e in certi campi riuscivo abbastanza bene
poi alla maturità ci hanno rifilato un problema di geometria che non aveva una soluzione 
Nonostante ciò sono uscito abbastanza bene
e poi all'Uni ho avuto ancora da superare esami di chimica, biochimica, fisica e statistica e anche lì sono uscito abbastanza bene
tuttavia, oggi preferisco i "pratici", o i divulgatori come ad es. Piero Angela, i quali spiegavano benissimo le cose, anche quelle più complesse, senza usare formule

|
|
|
sent on 07 Aprile 2024 (12:27) | This comment has been automatically translated (show/hide original)
Undoubtedly, :-D Indubbiamente |
|

Publish your advertisement on JuzaPhoto (info) |




 JuzaPhoto contains affiliate links from Amazon and Ebay and JuzaPhoto earn a commission in case of purchase through affiliate links.
JuzaPhoto contains affiliate links from Amazon and Ebay and JuzaPhoto earn a commission in case of purchase through affiliate links.